Molar Mass of H2
Gas has greater entropy than liquid and liquid has question_answer. To calculate the molar ratios you put the moles of one reactant over the moles of the other reactant.

Molar Mass Molecular Weight Of H2 Hydrogen Gas Youtube
Type the number of Kilogram per mole kgmol you want to convert in the text box to see the results in the table.
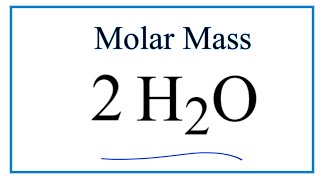
. Gram per mole gmol-Kilogram per mole kgmol- Standard atomic weight. To calculate the limiting reagent enter an equation of a chemical reaction and press the Start button. Atomic MassCreated By Tierra Keeling.
Molar masses of O 2 H 2 F 2 and N 2 are 32g 2016g 38g and 28012g respectively. Which species in the following reaction acts as a Lewis acid. Science Chemistry Chemistry Chemical Reactivity The molecule which contains largest number of molecules is to be identified from the given options.
You can determine the molecular weight by looking up the numbers under the element names in the periodic table. It also belongs to the class of dipolar aprotic solvents such as dimethylformamide and dimethyl sulfoxideIt is used in the petrochemical and plastics. Solution for The total mass of a solution is 1590 g.
2 mol -2418 kJmol-4836 kJ. The reactants and products along with their coefficients will appear above. The value is given in gmole or gramsmole.
FeCl2s H2g -. The molar mass of zinc nitrate will be equal to 1 atom x 65 gramsmole of zinc two atoms x 14 gramsmole of nitrogen six atoms x 16 gramsmole of oxygen 189 gramsmole of zinc nitrate. For all other compounds the general idea is the same.
Kilogram per mole kgmol - Molar mass units molar mass. Hydrogen H-Oxygen O-Sulfur S-Chlorine Cl-Iron Fe- Molecular mass. Calculate the molar ratios.
It is the formation of complex between the. The molar mass of a compound or an element is the mass per mole of the substance. This gives you a molar ratio of Al to I_2 of 0044480009456 Usually you divide each number in the fraction by the smaller number of moles.
The atomic mass of hydroxide rounding is 17008 atomic mass of oxygen 1600 atomic mass of hydrogen 1008. The conversion from Arachidonic acid to Prostaglandin H2 is a two step process. For example the chlorine molar mass is 3545 gramsmol.
N-Methyl-2-pyrrolidone NMP is an organic compound consisting of a 5-membered lactamIt is a colorless liquid although impure samples can appear yellow. CuSO4s 4 NH3aq CuNH342 A. First COX-1 catalyzes the addition of two free oxygens to form the 12-Dioxane bridge.
One molecule of water H 2 O would weigh 1802 amu 2100797 amu for H 159994 amu for O and a mole of water molecules would weigh 1802. You can also use our. This gives a ratio in which no number is less than 1.
Hoh Aqua Oh2 H₂O Oxidane Pure Water Hydroxic Acid Hydrogen Oxide H2O Molar Mass H2O Oxidation Number. What is the mass percent of the solute. Conversion formula for number of molecules from mass N u m b e r o f m o l e c u l e s M a s s i n g r a m s M o l a r.
ΣΔH f reactants ΣΔH f products so H2 O2 H2O is exothermic. 40 8. Basically you should know how to find the molar masses of any chemical compound now.
The solvent mass is 1252 g. It is synthesized from arachidonic acid in a reaction catalyzed by a cyclooxygenase enzyme. It is miscible with water and with most common organic solvents.
Prostaglandin H 2 is a type of prostaglandin and a precursor for many other biologically significant molecules.
How To Calculate The Molar Mass Of A Compound Quick Easy Youtube
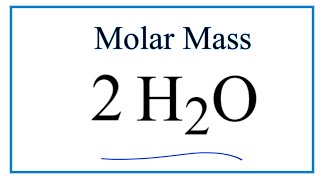
How To Calculate The Molar Mass Of 2h2o Youtube

Molar Mass Molecular Weight Of H2 Hydrogen Gas Youtube

How To Calculate The Molar Mass Of 2h2o Youtube

How To Find The Molar Mass Of H2s Hydrogen Sulfide Youtube
Hydrogen Molar Mass What S Insight
0 Response to "Molar Mass of H2"
Post a Comment